12. October 2023
35 hazardous chemicals added to the Prior Informed Consent Regulation (PIC)
Following an amendment to the EU’s Prior Informed Consent (PIC) Regulation. EU exporters are now obligated to notify their intentions...
Read More
14. August 2023 / EU REACH
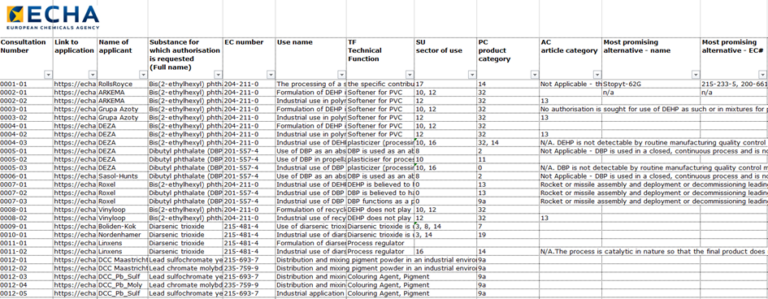
EU REACH Authorisation – Main Alternatives List
ECHA: Main alternatives to harmful substances subject to REACH authorisation Companies applying for an authorisation under REACH need to provide...
Read More
29. July 2023 / EU REACH
Formaldehyde restriction published
The restriction on formaldehyde and formaldehyde-releasing substances has been adopted by the Commission on July 14, 2023 and was published on July 17 in the OJEU: Regulation No. 2023/1464.
Read More
29. July 2023 / CLP
CLP Regulation Update 07/2023
Two regulations amending Annex VI to the CLP, Regulations 2023/1434 and 2023/1435, were published in the Official Journal on 11 July and enter into force on 31 July 2023.
Read More
19. July 2023
RESOLUTION dated 2023 No. “On Approval of the Regulation on State Registration of Disinfectants”- Ukraine
THE CABINET OF MINISTERS OF UKRAINE RESOLUTION dated 2023 No. Kyiv "On Approval of the Regulation on State Registration of...
Read More
7. July 2023
New EU chemicals enforcement project to focus on products sold online
The European Chemicals Agency's (ECHA) Enforcement Forum has announced two major projects to ensure compliance with regulations for products sold...
Read More
18. June 2023 / EU REACH, REACH authorisation
New two hazardous chemicals added to EU REACH Candidate List
ECHA has added two new chemicals to the Candidate List.
One is toxic for reproduction and the other has very persistent and very bioaccumulative hazardous properties. They are used, for example, in inks and toners and in the production of plastic products.
Entries added to the Candidate List on 14 June 2023: Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide; Bis(4-chlorophenyl) sulphone.
Read More
16. June 2023 / CLP
Industrial Use Mixtures – duty to PCN notification – the upcoming compliance date of January 2024
The upcoming compliance date of January 2024 for industrial use only mixtures necessitates adherence to harmonized information requirements outlined in Annex VIII to the CLP Regulation. Companies must ensure compliance and utilize available tools and support to facilitate successful notification. The report emphasizes the distinction between industrial use only mixtures and industrial use mixtures, the transitional period, the role of UFIs, and the need for accurate product categorization as per the EuPCS.
Read More
21. April 2023
NEW HAZARD CLASSES for substances and mixtures IN EU
EU has published a Delegated Regulation amending CLP Regulation, which sets out new hazard classes and criteria for the classification,...
Read More
12. April 2023 / REACH authorisation
Authorisation List – ECHA recommends eight substances for REACH authorisation
European Chemicals Agency (ECHA) has recommended eight substances, including lead, for addition to the REACH Authorization List
Read More