A Comprehensive Guide to SBP Authorization and Evaluation
Unveiling the Key Principles of Same Biocidal Product Regulation:
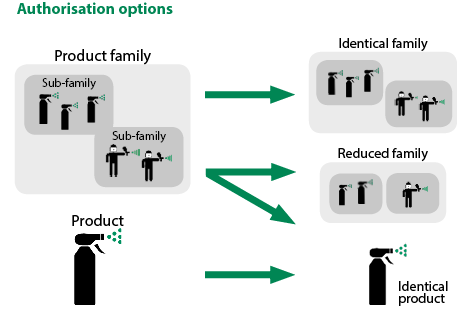
The following are the basic principles of the new Same Biocidal Product Regulation No 414/2013 (SBP regulation) as amended by Regulation No 2016/1802:
- Further SBP authorization can be granted by evaluating a previously authorized BP or one registered under BPD 98/8/EC 169. , or
- already authorised under the Biocidal Product Regulation No 170 528/2012 (BPR),
- or Applications can be requested for authorisations of SBP where there is already an identical product authorised
- or where the identical product is under evaluation and not yet authorised. The BP already authorised or under evaluation to be authorised is called the ‘related reference product’.(Or the reference BP). The precondition for authorisation of same BP is that these products are identical within the limited variations of an administrative 171 change . The terms and conditions for the SBP authorisation will be based on the evaluation made on the reference BP. The same rules mentioned above for a single BP apply also for a biocidal product family (BPF). Same product authorisation can also be granted for an individual product of a BP family.