EU has published a Delegated Regulation amending CLP Regulation, which sets out new hazard classes and criteria for the classification, labelling and packaging of substances and mixtures.
It applies to all chemical substances and mixtures placed on the EU market under REACH. It also applies to active substances in biocidal products. and plant protection products, which are normally prioritised for harmonised classification in the EU.
ECHA provides advice on new hazard classes for substances and mixtures
Three new hazard classes for classifying, labelling and packaging (CLP) substances and mixtures enter into force on 20 April 2023. ECHA has published information on the application dates and related guidance.
The European Commission has updated the Classification, Labelling and Packaging Regulation with the following hazard classes:
- endocrine disruptors (ED) for human health or the environment;
- persistent, bioaccumulative and toxic (PBT); very persistent and very bioaccumulative (vPvB); and
- persistent, mobile and toxic (PMT); very persistent and very mobile (vPvM).
Companies and Member State authorities can use current guidance on identifying endocrine disruptors and. on PBT (persistence, bioaccumulation, toxicity) assessment until the guidance on applying the CLP criteria has been updated. It is expected to be ready in 2024. and make their dossiers comply with the information requirements, where needed.
What are the obligations for companies following the entry into force of the Commission Delegated Regulation (EU) 2023/707 of 19 December 2022?
Weblink: https://qnapublic.echa.europa.eu/?ids=2006
Answer:
Once the Delegated Regulation 2023/707 enters into force (20 April 2023), manufacturers, importers, downstream users and distributors should consider the following tasks in order to comply with their duties. The exact order and timing will depend on the specific scenario of each operator: if they place on the market substances or mixtures; if they are members of a joint submission or not; if their substance or mixture is already placed on the market or not, etc. The list is not exhaustive but lists the main tasks triggered by the new legal text.
Review the classification and labelling of the substances and mixtures in their portfolio against the criteria of the new hazard classes. They should use all available information for self-classification.
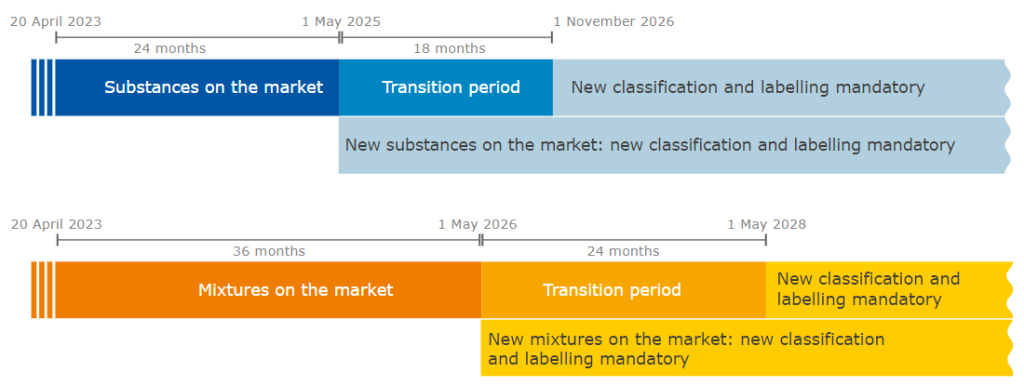
Take note of the transitional periods for substances and mixtures that apply to them and make. sure they have fulfilled their duties at the latest by those dates. Transitional periods allow to comply earlier and avoid last-minute difficulties.
If they are part of a joint submission under REACH, initiate the discussion to agree on the classification and labelling of their substances. Update the registration dossier without undue delay, as outlined in the Implementing Regulation 2020/1435.
If they are part of a group of manufacturers/importers for the purpose of C&L notification, initiate the discussion to agree on the classification and labelling of their substances. Update the notification to the inventory accordingly.
Contact the other notifiers for the same substance to agree on the new classification and labelling. The leader of the joint submission, or the leader of the group of manufacturers/importers, if the operator is a member of either, can help in such communication.
Revise their labels, for both substances and mixtures.
Update, when relevant, any poison centre notification they have already made, with the new hazard information.
Consider whether a new poison centre notification is needed. Mixtures previously not classified for human health nor physical hazards may have to be classified for the new human health hazards from 1 May 2026.
Update their safety data sheets (SDS) with the new classification and labelling, and all relevant new information that is. needed to reflect the new hazards. They may need to compile an SDS for substances or mixtures which were previously not classified.
Prepare to compile and provide an SDS upon request if their mixtures are not classified but contain a substance in 0,1% or more of an endocrine disruptor Category 2 and is not intended for the general public.
Transitional periods:
Full article ECHA: https://echa.europa.eu/sk/-/echa-provides-advice-on-new-hazard-classes-for-substances-and-mixtures
Ekotox Centers EU SDS/CLP webpages: https://ekotox.eu/safety-data-sheet-sds/
Training: EU SDS Compliance *Webinar*: https://ekotoxtraining.com/events/eu-sds-compliance-webinar-2/
