COMMISSION REGULATION (EU) 2024/858 (March 14, 2024) of European Parliament and Council Regulation 1223/2009/EC on styrene/acrylate copolymer, sodium styrene/acrylate copolymer, copper, copper colloid, hydroxyapatite, on the modification of gold, gold colloid, gold thioethylaminohyaluronic acid, acetyl heptapeptide-9 gold colloid, platinum, platinum colloid, acetyl tetrapeptide-17 platinum colloid and silver colloid nanomaterials in cosmetic products
The investigation of the Scientific Committee for Consumer Safety on the safety of nanomaterials in cosmetic products states that the aforementioned nanomaterials may pose a health risk to consumers if they are used in cosmetic products.
Use of hydroxyapatite in cosmetic products
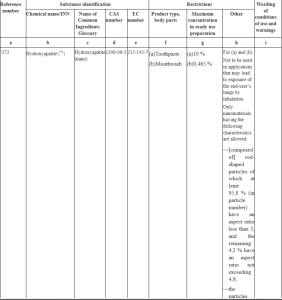
COMISSION (EU) 2024/858 REGULATION: Rendelet – EU – 2024/858 – EN – EUR-Lex (europa.eu)
Ekotox Cosmetics webpage: https://ekotox.eu/cosmetics-2/
