Safety data sheet (SDS)
- substances must be classified only according to CLP;
- the mixtures must be classified, labeled and packaged only according to CLP;
- substances and mixtures in safety data sheets must be classificated according to CLP.
EKOTOX CENTERS offers preparation of Safety Data Sheets and revisions under REACH/CLP in most of EU languages.
All safety data sheets provided after 31 December 2022 have to be in the format according to Regulation (EU) 2020/878.
The adoption of the new format from Regulation (EU) 2020/878 is advised for SDSs to comply by 31 December 2022.
The European Parliament and Council adopted the CLP Regulation in December 2008, ensuring effective chemical management. The purpose of CLP is to ensure a high level of protection of human health and the environment as well as the free movement of substances, mixtures and certain articles.
The CLP Regulation applies the terminology, evaluation principles and criteria of the GHS (Globally Harmonised System), and includes the provisions on the classification & labelling inventory from Regulation (EC) No 1907/2006 (REACH Regulation).
“All safety data sheets provided after 31 December 2022 have to be in the format according to Regulation (EU) 2020/878. It is recommended that the new format, as set out in Regulation (EU) 2020/878, is adopted, as soon as practicable, to ensure that all SDSs comply by the 31 December 2022 deadline.”
SAFETY DATA SHEETS – REACH requirements
- Article 31
Requirements for Safety Data Sheets
1. The supplier’s Obligation
The supplier of a substance or a preparation shall provide the recipient of the substance or preparation with a safety data sheet compiled in accordance with Annex II:
(a) where a substance or preparation meets the criteria for classification as dangerous in accordance with Directives 67/548/EEC or 1999/45/EC; or
(b) where a substance is persistent, bioaccumulative and toxic or very persistent and very bioaccumulative in accordance with the criteria set out in Annex XIII; or
(c) where a substance is included in the list established in accordance with Article 59(1) for reasons other than those referred to in points (a) and (b).
2. Consistency with Chemical Safety Assessment
Every supply chain actor performing a chemical safety assessment under Articles 14 or 37 must align safety data sheet details with their assessment. When a safety data sheet pertains to a preparation, and the actor has assessed its chemical safety, alignment with the preparation’s chemical safety report suffices, rather than each substance’s report.
3. Providing Safety Data Sheets on Request
The supplier shall provide the recipient at his request with a safety data sheet compiled in accordance with Annex II, where a preparation does not meet the criteria for classification as dangerous in accordance with Articles 5, 6 and 7 of Directive 1999/45/EC, but contains:
(a) in an individual concentration of ≥ 1 % by weight for nongaseous preparations and ≥ 0,2 % by volume for gaseous preparations at least one substance posing human health or environmental hazards; or
(b) in an individual concentration of ≥ 0,1 % by weight for non-gaseous preparations at least one substance that is persistent, bioaccumulative and toxic or very persistent and very bioaccumulative in accordance with the criteria set out in Annex XIII or has been included for reasons other than those referred to in point (a) in the list established in accordance with Article 59(1); or
(c) a substance for which there are Community workplace exposure limits.
4. Exemption for Publicly Offered Dangerous Substances
You don’t have to provide a safety data sheet if products for the public include enough info for health, safety, and environmental protection, except when asked by a distributor or user.
5. Language Provision for Safety Data Sheets
The safety data sheet shall be supplied in an official language of the Member State(s) where the substance or preparation is placed on the market, unless the Member State(s) concerned provide otherwise.
Commission Regulation 2024/2865
On November 20, 2024, the European Union published Commission Regulation 2024/2865 amended the EU CLP Regulation. The amendment took effect on December 10, with most provisions becoming mandatory from July 1, 2026 (18 months), and the remaining provisions applicable from January 1, 2027 (24 months).
COMMISSION DELEGATED REGULATION (EU) 2023/707 of 19 December 2022 amending Regulation (EC) No 1272/2008 as regards hazard classes and criteria for the classification, labelling and packaging of substances and mixtures
The European Commission released a Delegated Regulation that modifies the CLP Regulation. This regulation introduces fresh hazard classes and criteria for categorizing, labeling, and packaging substances and mixtures.
It applies to all chemical substances and mixtures placed on the EU market under REACH. This principle extends to active agents in biocidal and plant protection products, typically prioritized for harmonized EU classification.
This EU legislation is binding to manufacturers, importers, downstream users and distributors placing substances on the European Union market. Member States will also refer to the new hazard classes and criteria when making proposals for harmonised classification and labelling.
The new hazard classes are:
- ED HH in Category 1 and Category 2 (Endocrine disruption for human health)
- ED ENV in Category 1 and Category 2 (Endocrine disruption for the environment)
- PBT (persistent, bioaccumulative, toxic), vPvB (very persistent, very bioaccumulative)
- PMT (persistent, mobile, toxic), vPvM (very persistent, very mobile)
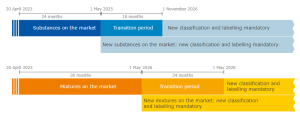
Application dates
The new rules are in force as of 20 April 2023. Beginning today, Member States can propose harmonised classification and labelling (CLH) involving new hazard classes. Meanwhile, manufacturers, importers, downstream users, and distributors can autonomously classify their substances and mixtures.
Following the enforcement of the Delegated Regulation, transitional periods come into play. Manufacturers, importers, and distributors are not required to classify substances right away. Instead, they may choose to voluntarily apply new hazard classes.
At the end of the transitional periods, all manufacturers, importers, downstream users and distributors must apply the new hazard classes.
Effective Communication Obligations Along the Supply Chain for Substances and Mixtures Without Mandatory Safety Data Sheets
1. Any supplier of a substance on its own or in a preparation who does not have to supply a safety data sheet in accordance with Article 31 shall provide the recipient with the following information:
(a) the registration number(s) referred to in Article 20(3), if available, for any substances for which information is communicated under points (b), (c) or (d) of this paragraph;
(b) if the substance is subject to authorisation and details of any authorisation granted or denied under Title VII in this supply chain;
(c) details of any restriction imposed under Title VIII;
(d) any other available and relevant information about the substance that is necessary to enable appropriate risk management measures to be identified and applied including specific conditions resulting from the application of Section 3 of Annex XI.
2. The information referred to in paragraph 1 shall be communicated free of charge on paper or electronically at the latest at the time of the first delivery of a substance on its own or in a preparation after 1 June 2007.
3. Suppliers shall update this information without delay on the following occasions:
(a) as soon as new information which may affect the risk management measures, or new information on hazards becomes available;
(b) once an authorisation has been granted or refused;
(c) once a restriction has been imposed.
EKOTOX CENTERS are providing consultancy services and customers support – Safety Data Sheets authoring, revisions, complex management, Legal Compliance Services, PCN and UFI services and more.
EKOTOX CENTERS – Contact us: https://ekotox.eu/about-company/contact-form/
Safety Data Sheet
- Safety Data Sheet – Definition
- Why is SDS needed?
- Format of safety data sheet
- Update of safety data sheet
- Labels design
- How will CLP affect the SDS?
- Content of safety data sheet
- The content of an SDS – Responsibility
- Who should compile an safety data sheet (SDS)
- SDS and label