Ecotox Centers are offering consultation services focused on REACH legislation, Management of SVHC substances, communication in supply chain and support for EU chemicals legislation compliance.
We do have done several projects in terms of:
- REACH Authorisation applications – development of whole REACH Authorisation documentation.
- Downstream users compliance – Internal Documentation for REACH Authorisation Compliance (IDRAC).
If you have questions please contact us:
phone +421 2 45943712 / e-mail ekotox(at)ekotox.eu
FB: Ekotox Centers
Twitter: Ekotox Centers
Linkedin: Ekotox
REACH Authorisation Services:
-
REACH Authorisation Application
Whole management, coordination and elaboration of the Application for REACH Authorisation:
- Development of application strategy;
- Chemical Safety Report;
- Analysis of Alternatives;
- Socio-economic Analysis;
- Substitution Plan.
-
REACH Authorisation/SVHCs Screening/Audit
Companies (producers / importers) now need to understand the status of SVHCs use in their products portfolio – mixtures and products (articles) entering EU market. That means also to check the suppliers for their compliance with EU chemicals legislation.
- Inventory of SVHCs use and their status;
- SVHCs analysis for general requirements, SCIP and more;
- Development of the „corporate“ strategy;
- Internal rules and application manuals development;
- Communication Strategy;
- SVHCs Application Plan.
-
Downstream User Compliance Screening/Audit
Companies (downstream users) are under the preasure of the additional requirements to understand the status of SVHCs use. Having SVHCs in their products portfolio means the risk of additional administrative burden as well as rising prices of supplies affected by REACH authorisation. Additional costs for REACH authorisation complience as well as risk that such a material disapear are quite challenging.
- Inventory of SVHCs use and their status;
- Annex XIV substances use and their status;
- Development of the – Internal Documentation for REACH Authorisation Compliance (IDRAC);
- Communication and Implementation Strategy;
REACH Authorisation Process
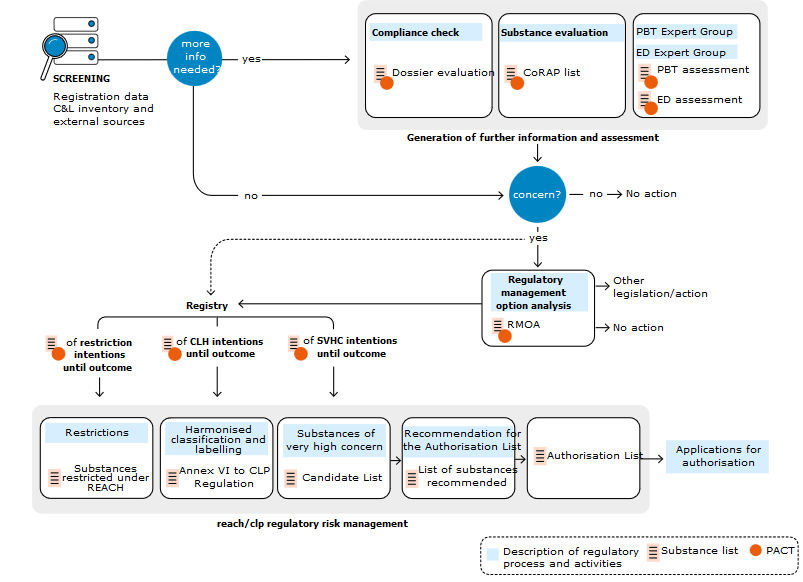
The authorisation process is described in the scheme below:
Link to ECHA: https://echa.europa.eu/substances-of-potential-concern
REACH Authorization is related to substances listed in the Annex XIV of the REACH regulation – “Authorisation list”.
In fact it means a complete ban on the use of chemical substances except “Authorised use” – Decision on authorisation issued by the European Commission.
Authorisation process require the preparation and submission of documentation proving that the use is safe; furthermore, it currently cannot be replaced. Specific dates are determined for submission of documentations as well as sunset day for application.
Criteria for Granting REACH Authorizations
Manufacturers, importers, or downstream users seeking authorization for a substance on the Authorisation List can apply.
To obtain authorization, applicants must demonstrate controlled risk. If not, socio-economic benefits can outweigh risks, allowing authorization despite lacking alternatives.
The application includes chemical safety info, alternative analysis, and substitution plans. It might have a socio-economic assessment.
Once the application and fee are received, ECHA’s RAC and SEAC review it. They ensure it complies with REACH’s info requirements (Article 62). Additional data might be asked for.
Authorization serves as a risk management tool for chemicals, safeguarding health and the environment. It targets substances with *CMR, *PBT, and *vPvB attributes, backed by scientific evidence indicating significant potential harm. Safer alternatives should replace these substances.
- CMR (carcinogenic, mutagenic or toxic for reproduction) – substances that meet the criteria for classification in category 1 and 2 according to Directive 67/548/EEC.
- PBT (persistent, bioaccumulative and toxic) or vPvB (very persistent and very bioaccumulative) – substances that meet the criteria set out in Annex XIII of the REACH Regulation.
- Substances for which there is scientific evidence of probable serious effects to human health or the environment. Which give rise to the same level of concern as listed substances (i.e. substances with endocrine disruptors or those having persistent, bioaccumulative and toxic properties or very persistent and very bioaccumulative properties, which do not meet the criteria in Annex XIII of the REACH Regulation.
A downstream user can utilize an authorized substance as permitted. They must source it from the authorized company, adhere to stated conditions, and inform ECHA of usage. Additionally, they can seek their own authorization, a process demanding time for communication, cooperation, and decision-making.
Please contact us:
phone +421 2 45943712 / e-mail ekotox(at)ekotox.eu
FB: Ekotox Centers
Twitter: Ekotox Centers
Linkedin: Ekotox
Authorisation: